Why Organize Elements in a Table?
The arrangement of chemical elements in the modern periodic table has become as recognizable as a world map, but this clarity wasn't always evident.
Dmitri Mendeleev, the architect of the periodic table, embarked on this task in 1869. He methodically compiled and categorized the established attributes of elements during his train journeys, almost akin to playing a strategic game. Amidst this process, he identified clusters of elements that displayed analogous characteristics. However, he also observed numerous deviations from the emerging trends.
Remarkably, instead of relinquishing his efforts, he opted to adjust the recorded property values to align more cohesively with the developing patterns. Additionally, he ventured to hypothesize the existence of certain elements that were absent at that time, all in an earnest endeavor to harmonize the patterns within his conceptual "game."
Despite encountering considerable skepticism and enduring years of efforts to gain global endorsement, Mendeleev's principles ultimately prevailed. The validation came when newly discovered elements aligned with his predictions, solidifying the credibility of his patterns. Furthermore, some of the properties he had creatively manipulated were subsequently reevaluated and found to closely correspond with his projections.
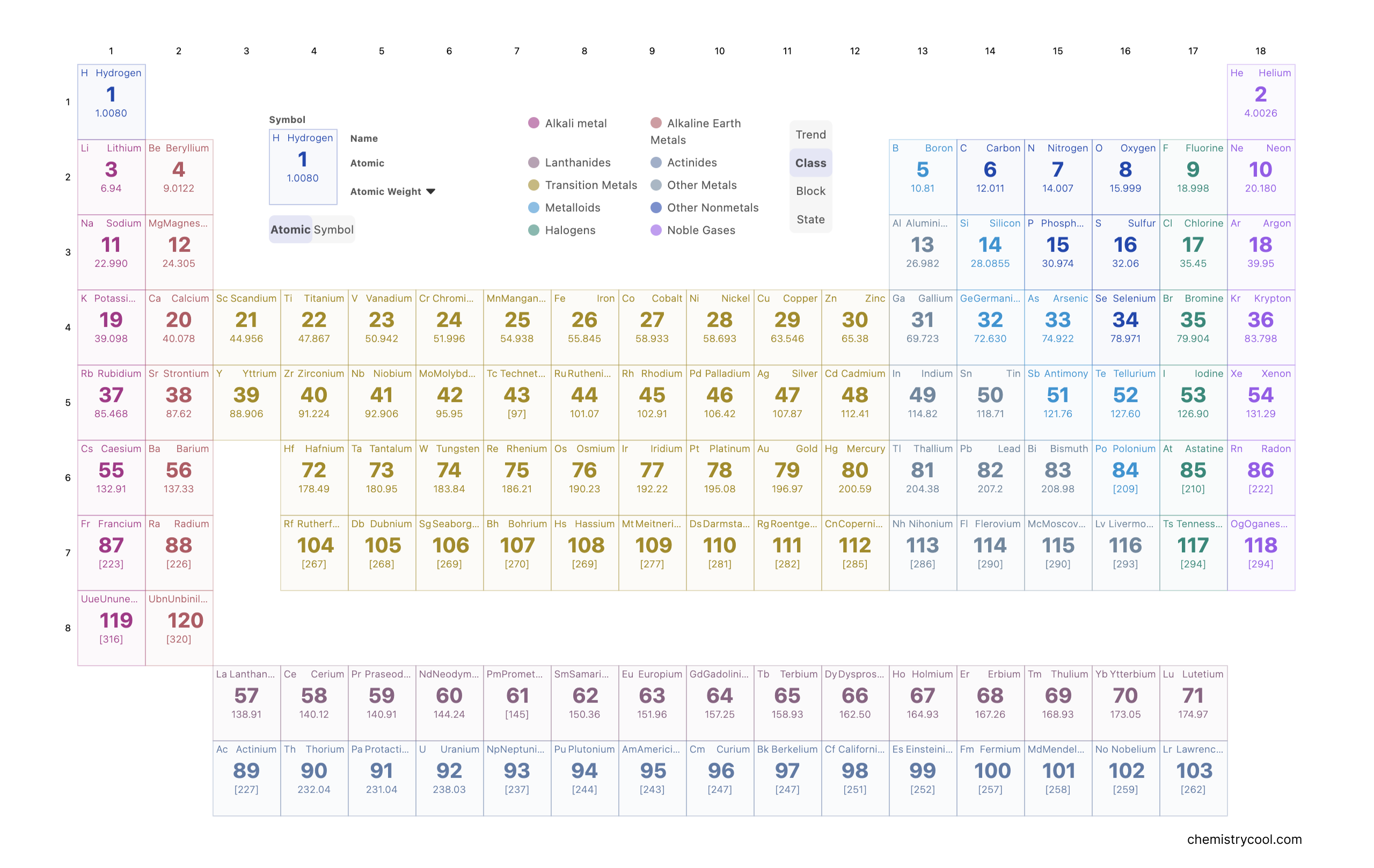
What groups on the periodic table ?
In Periodic Table, a group refers to a vertical column within the arrangement of chemical elements in the periodic table. Within a group, these chemical elements possess atoms that share the same count of valence electrons as well as an identical count of valence vacancies. This commonality in both the configuration and structure of their atomic valence shells suggests a corresponding similarity in their physical and chemical characteristics. The groups are sequentially numbered from 1 to 18.
When observing the periodic table from left to right, we encounter two sets of elements (groups 1 and 2) located in the s-block, also known as the hydrogen block. Moving along, there are ten groupings (numbered 3 through 12) situated in the d-block, recognized as the transition block. Following these, we find six groups (numbered 13 through 18) in the p-block, designated as the main block. It is noteworthy that the elements positioned in the f-block, specifically the lanthanoids and actinoids constituting the inner-transition block, do not possess designated group numbers.
What periodic table periods ?
A period within the periodic table is a horizontal arrangement. There are precisely seven periods, each commencing from the far left side. The inception of a new period takes place when a fresh principal energy level starts to accommodate electrons. The initial period, Period 1, contains merely two elements: hydrogen and helium. Subsequently, both periods 2 and 3 encompass a total of 8 elements each. Moving ahead, periods 4 and 5 comprise 18 elements. For periods 6 and 7, this number expands to 32 elements, attributed to the inclusion of two distinct bottom rows that are set apart from the main table structure.
Periodic table with atomic number
The elements in the modern periodic table, are arranged based on their ascending atomic numbers. The atomic number signifies the count of protons housed within an atom's nucleus. This count of protons distinctly identifies an element (for example, an element possessing 6 protons is invariably a carbon atom, regardless of the potential variation in neutron count). The proton count also dictates the quantity of electrons orbiting the nucleus. Consequently, the disposition of these electrons governs the majority of an element's chemical characteristics.
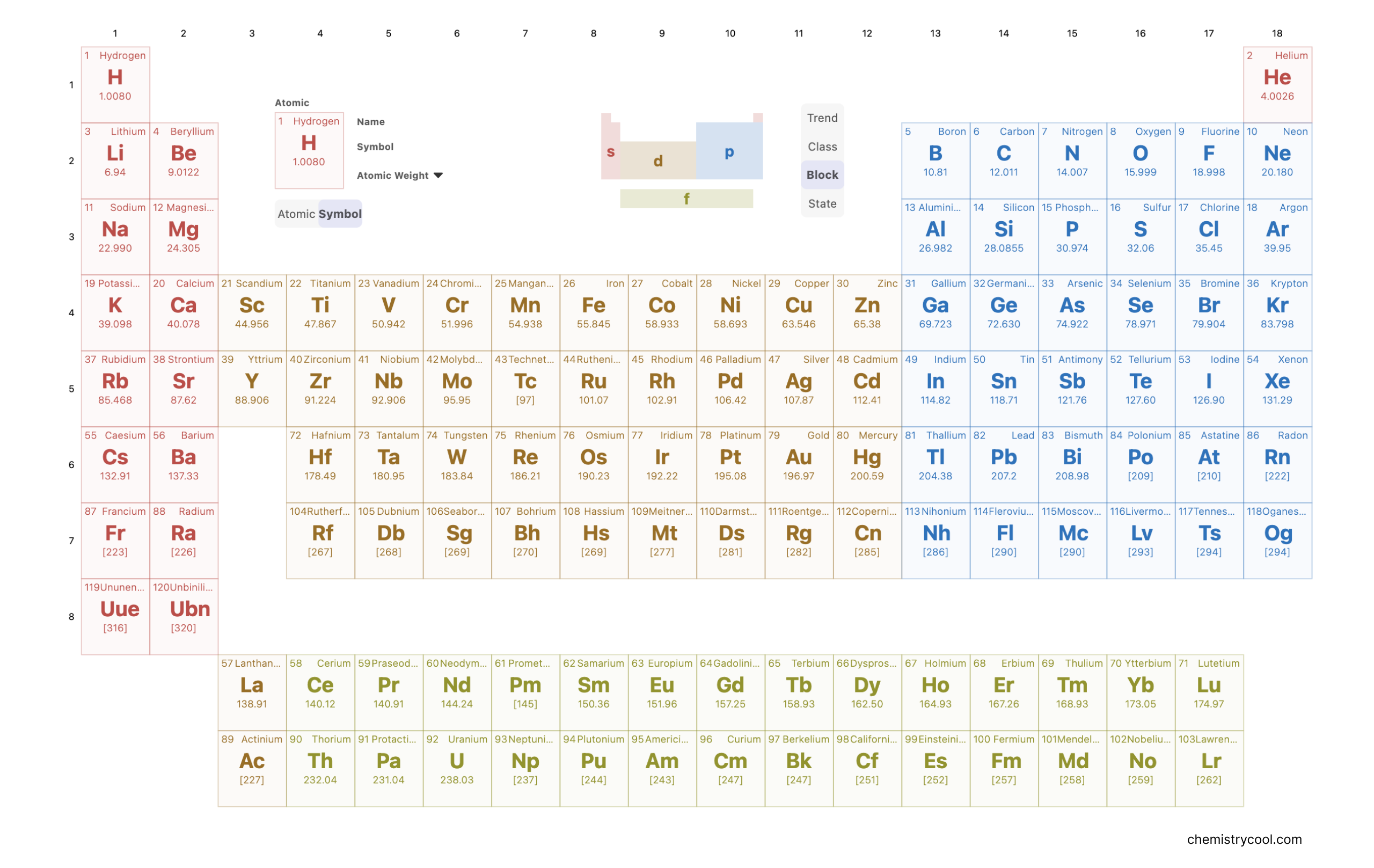
Periodic table blocks
The periodic table is organized into distinct blocks, determined by the types of electron shells being filled: s, p, d, and f blocks, encompassing the entire table. The introduction of the term "block" can be credited to Charles Janet, who first used it when unveiling his left step periodic table (LSPT). These blocks are defined by their unique properties, as exemplified by:
The s-block, comprising highly electropositive metals in addition to helium and hydrogen.
The p-block, encompassing metals, non-metals, and metalloids within groups 3 to 8.
The d-block, including metals with variable (transition) oxidation states.
The f-block, consisting of Lanthanide and Actinide metals.

 Blank
Blank Atomic Weight
Atomic Weight